Cellution Biologics Inc. Announces First Peer-Reviewed Publication Demonstrating Positive Outcomes for AmchoPlast™ in the Treatment of Chronic Diabetic Foot Ulcers
ROSWELL, Ga., Dec. 16, 2025 (GLOBE NEWSWIRE) -- Cellution Biologics, Inc., a leader in regenerative allograft solutions, proudly announces the publication of its first peer-reviewed clinical article, “Bridging the Wound Gap: Interim Results from Randomized Trials Evaluating Dehydrated Human Amnion-Intermediate Layer-Chorion Membrane for the Treatment of Non-healing Diabetic Foot Ulcers,” published in Cureus. This milestone marks a significant achievement for the company and reinforces its commitment to advancing evidence-based medicine.
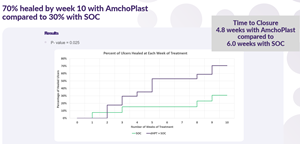
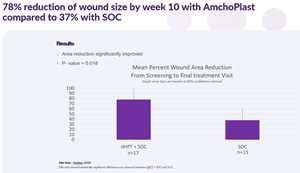
The publication presents interim results from two prospective, randomized, multicenter clinical studies evaluating AmchoPlast™, Cellution Biologics’ dehydrated human amnion-intermediate layer-chorion membrane (dHAICM) allograft, as an adjunct to standard of care for patients with chronic, non-healing diabetic foot ulcers. The findings demonstrate a statistically significant improvement in complete wound closure rates when AmchoPlast™ is added to standard of care. In the ongoing ELITE study, 71% of patients receiving AmchoPlast™ plus standard of care achieved full healing compared to 23% receiving standard of care alone.
“These results reflect the real-world impact our offerings can have on patients and providers managing the most challenging chronic wounds,” said Shiva Arjunon, President of Cellution Biologics Inc. “This first publication is not only a scientific milestone for Cellution Biologics Inc., but also a meaningful validation of our mission to bring high-quality, clinically supported allograft solutions to healthcare professionals.”
This marks the first of several clinical publications currently underway across multiple therapeutic areas, including advanced wound care, burn reconstruction, soft tissue repair, and ocular applications. The growing body of research underscores Cellution Biologics Inc.’s long-term commitment to rigorous clinical validation, scientific transparency, and innovation.
“We are deeply proud of this achievement, and even more proud of what it represents—a sustained investment in clinical evidence and a dedication to improving outcomes for patients,” said Shiva Arjunon. “Our team, clinical partners, and investigators have worked tirelessly to ensure that our products are backed by strong scientific data.”
As the healthcare landscape increasingly emphasizes evidence-based medicine, Cellution Biologics Inc. remains committed to supporting clinicians with high-quality data, meaningful scientific contributions, and biologic solutions designed to improve patient care.
About Cellution Biologics Inc.
Cellution Biologics Inc. is a regenerative medicine company specializing in advanced human tissue–based allografts for surgical, wound care, and specialty clinical applications. With a focus on scientific rigor, innovation, and quality, Cellution Biologics Inc. partners with clinicians to deliver solutions that enhance healing while supporting operational excellence across diverse care settings.
Media Contact:
Jon Werner | Vice President – Marketing | Cellution Biologics Inc
jon.w@cellutionbiologics.com | +1 443 315 3344 | cellutionbiologics.com
Photos accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/1d3d06d0-663d-442b-99ac-ab0599f5c3d1
https://www.globenewswire.com/NewsRoom/AttachmentNg/0d8785b5-67da-4c98-b87a-456eae93e94b

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.